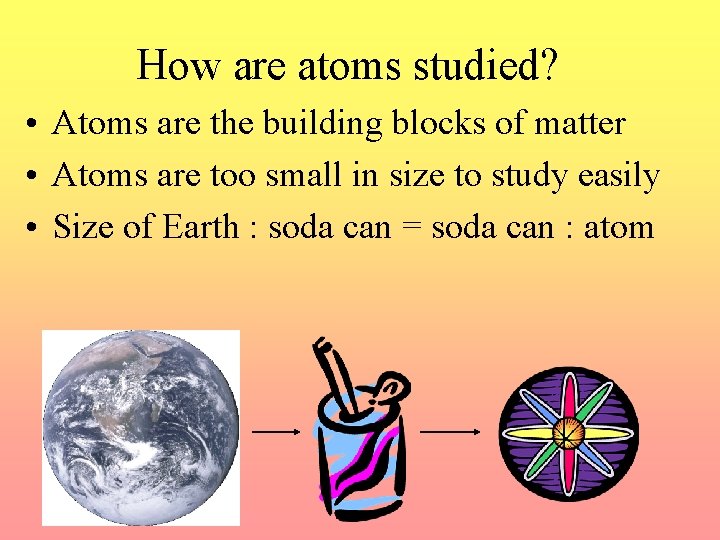
Atoms are electrically neutral if they have an equal number of protons and electrons. Atoms that have either a deficit or a surplus of electrons are called ions. Electrons that are farthest from.
Take anything apart andyou'll find something smaller inside. There are engines inside cars,pips inside apples, hearts and lungs inside people, and stuffinginside teddy bears. But what happens if you keep going? If you keeptaking things apart, you'll eventually, find that allAtoms are the basic unit of chemistry. They consist of 3 smaller things: Protons - these are positively charged (+) Electrons - these are negatively charged (-). Atoms with the same atomic number but a different mass number are isotopes. List the isotopes of hydrogen and of carbon. Be able to describe radioisotopes and list three ways they are used in biology. The union between the electron structures of atoms is known as the chemical bond.
matterAn atom is the smallest unit of ordinary matter that forms a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. Atoms are comprised of even smaller particles called neutrons, protons and electrons. The neutrons and protons have positive charges and are very large and heavy compared to the electrons. These two particles exist in the nucleus of the atom, which is located at its center.
(all the 'stuff' that surrounds us) is made fromdifferent typesof atoms. Living things, for example, are mostly made from the atomscarbon, hydrogen, and oxygen. These are just three of over 100chemical elements that scientists havediscovered. Otherelements include metals such as copper, tin, iron andgold, and gaseslike hydrogen and helium. You can make virtually anything you canthink of by joining atoms of different elements together like tinyLEGO®blocks.Once the way atoms are put together is understood, the question of how they interact with each other can be addressed—in particular, how they form bonds to create molecules and macroscopic materials. There are three basic ways that the outer electrons of atoms can form bonds:

Atoms Are Indivisible
- Electrons can be transferred from one atom to another.
- Electrons can be shared between neighbouring atoms.
- Electrons can be shared with all atoms in a material.
The first way gives rise to what is called an ionic bond. Consider as an example an atom of sodium, which has one electron in its outermost orbit, coming near an atom of chlorine, which has seven. Because it takes eight electrons to fill the outermost shell of these atoms, the chlorine atom can be thought of as missing one electron. The sodium atom donates its single valence electron to fill the hole in the chlorine shell, forming a sodium chloride system at a lower total energy level.
An atom that has more or fewer electrons in orbit than protons in its nucleus is called an ion. Once the electron from its valence shell has been transferred, the sodium atom will be missing an electron; it therefore will have a positive charge and become a sodium ion. Simultaneously, the chlorine atom, having gained an extra electron, will take on a negative charge and become a chlorine ion. The electrical force between these two oppositely charged ions is attractive and locks them together. The resulting sodium chloride compound is a cubic crystal, commonly known as ordinary table salt.
The second bonding strategy listed above is described by quantum mechanics. When two atoms come near each other, they can share a pair of outermost electrons (think of the atoms as tossing the electrons back and forth between them) to form a covalent bond. Covalent bonds are particularly common in organic materials, where molecules often contain long chains of carbon atoms (which have four electrons in their valence shells).
Finally, in some materials each atom gives up an outer electron that then floats freely—in essence, the electron is shared by all of the atoms within the material. The electrons form a kind of sea in which the positive ions float like marbles in molasses. This is called the metallic bond and, as the name implies, it is what holds metals together.
Atoms Are The Smallest Particles Of Matter
There are also ways for atoms and molecules to bond without actually exchanging or sharing electrons. In many molecules the internal forces are such that the electrons tend to cluster at one end of the molecule, leaving the other end with a positive charge. Overall, the molecule has no net electric charge—it is just that the positive and negative charges are found at different places. For example, in water (H2O) the electrons tend to spend most of their time near the oxygen atom, leaving the region of the hydrogen atoms with a positive charge. Molecules whose charges are arranged in this way are called polar molecules. An atom or ion approaching a polar molecule from its negative side, for example, will experience a stronger negative electric force than the more-distant positive electric force. This is why many substances dissolve in water: the polar water molecule can pull ions out of materials by exerting electric forces. A special case of polar forces occurs in what is called the hydrogen bond. In many situations, when hydrogen forms a covalent bond with another atom, electrons move toward that atom, and the hydrogen acquires a slight positive charge. The hydrogen, in turn, attracts another atom, thereby forming a kind of bridge between the two. Many important molecules, including DNA, depend on hydrogen bonds for their structure.
Atoms Are Composed Of
Finally, there is a way for a weak bond to form between two electrically neutral atoms. Dutch physicist Johannes van der Waals first theorized a mechanism for such a bond in 1873, and it is now known as van der Waals forces. When two atoms approach each other, their electron clouds exert repulsive forces on each other, so that the atoms become polarized. In such situations, it is possible that the electrical attraction between the nucleus of one atom and the electrons of the other will overcome the repulsive forces between the electrons, and a weak bond will form. One example of this force can be seen in ordinary graphite pencil lead. In this material, carbon atoms are held together in sheets by strong covalent bonds, but the sheets are held together only by van der Waals forces. When a pencil is drawn across paper, the van der Waals forces break, and sheets of carbon slough off. This is what creates the dark pencil streak.




