Commonly Tested Lewis Structures
- Read my article in Science Education based on my dissertation.
We draw Lewis Structures to predict:
-the shape of a molecule.
-the reactivity of a molecule and how it might interact with other molecules.
-the physical properties of a molecule such as boiling point, surface tension, etc.
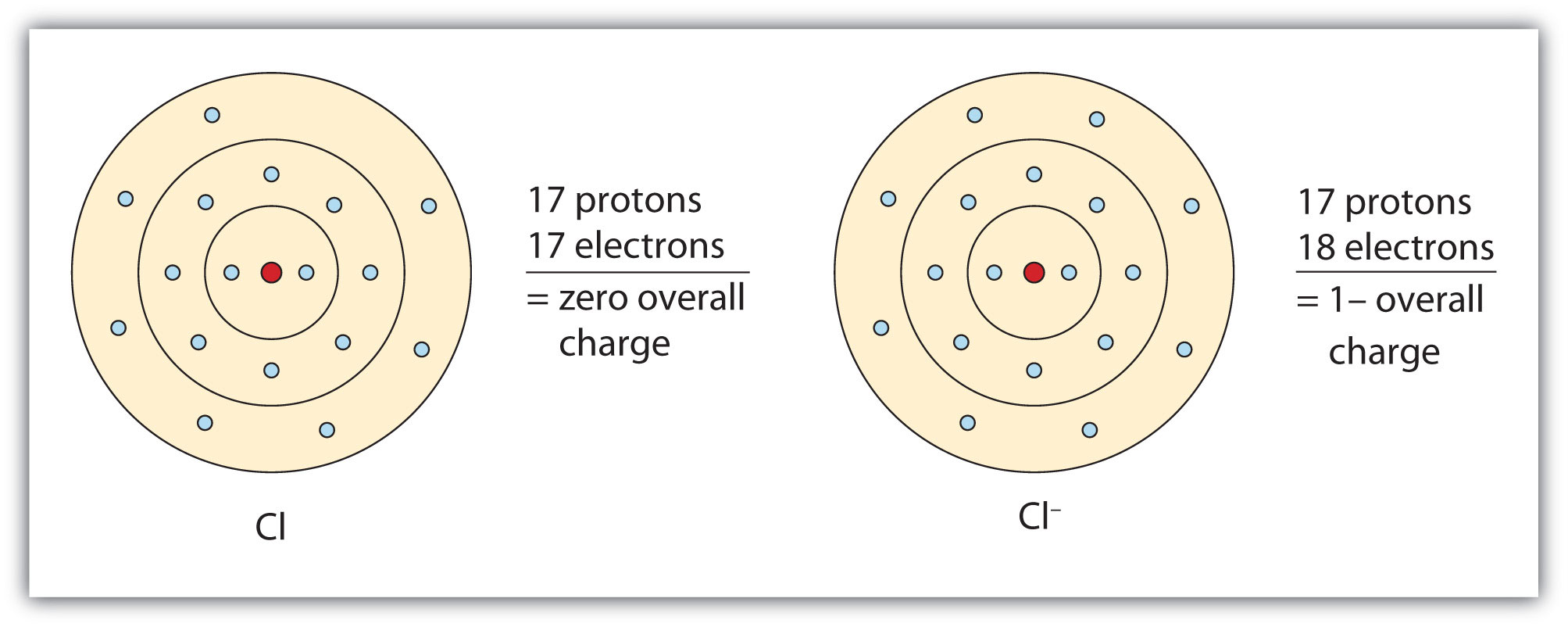
An atom such as chlorine (Cl), that contains seven electrons in its valence shell, needs one more electron to have a full valence shell. If we use the same logic as we did for sodium, we should conclude that chlorine would rather gain one more electron than lose all seven of its valence electrons to achieve stability. In this step,we will find out the valence electrons of chlorine.We have to know that the electrons of valence shell are called valence electrons. Cl (17)=1s²2s²2p⁶3s²3p⁵ From the above electron configuration of chlorine,we see that chlorine has 7 valence electrons in its valence shell.
Video: Drawing the Lewis Structure for ClO2-
For the ClO2- Lewis structure the total number of valence electrons (found on the periodic table) for the ClO2- molecule. Once we know how many valence electrons there are in ClO2- we can distribute them around the central atom with the goal of filling the outer shells of each atom.
In the Lewis structure for ClO2- we put Chlorine (Cl) at the center of the structure since it is the least electronegative. There are total of 20 valence electrons for the ClO2- Lewis structure. Remember that the negative sign counts as one valence electron.
To show that the ClO2- Lewis structure is an ion with a -1 change we need to put brackets around the structure and put a negative side on the outside of the brackets.
Cl Valence Electrons
It is helpful if you:
- Try to draw the ClO2- Lewis structure before watching the video.
- Watch the video and see if you missed any steps or information.
- Try structures similar to ClO2- for more practice.
List of Lewis Structures
| Acetone | BF3 | BH4- | BrF5 | BrO3- | C2H2 | C2H4 | ClO- |
| CH4 | ClO2- | ClO2 | ClO4- | CO | CS2 | H2O | H3O+ |
| HCl | HNO3 | I3- | ICl4- | IF5 | N2 | N3- | NH2OH |
| NH3 | NO2- | NO2 | NO3- | O2 | OF2 | PCl5 | PH3 |
| PO33- | PO43- | SCl2 | SF4 | SF6 | SO3 | SO42- | XeF2 |
| XeF4 |

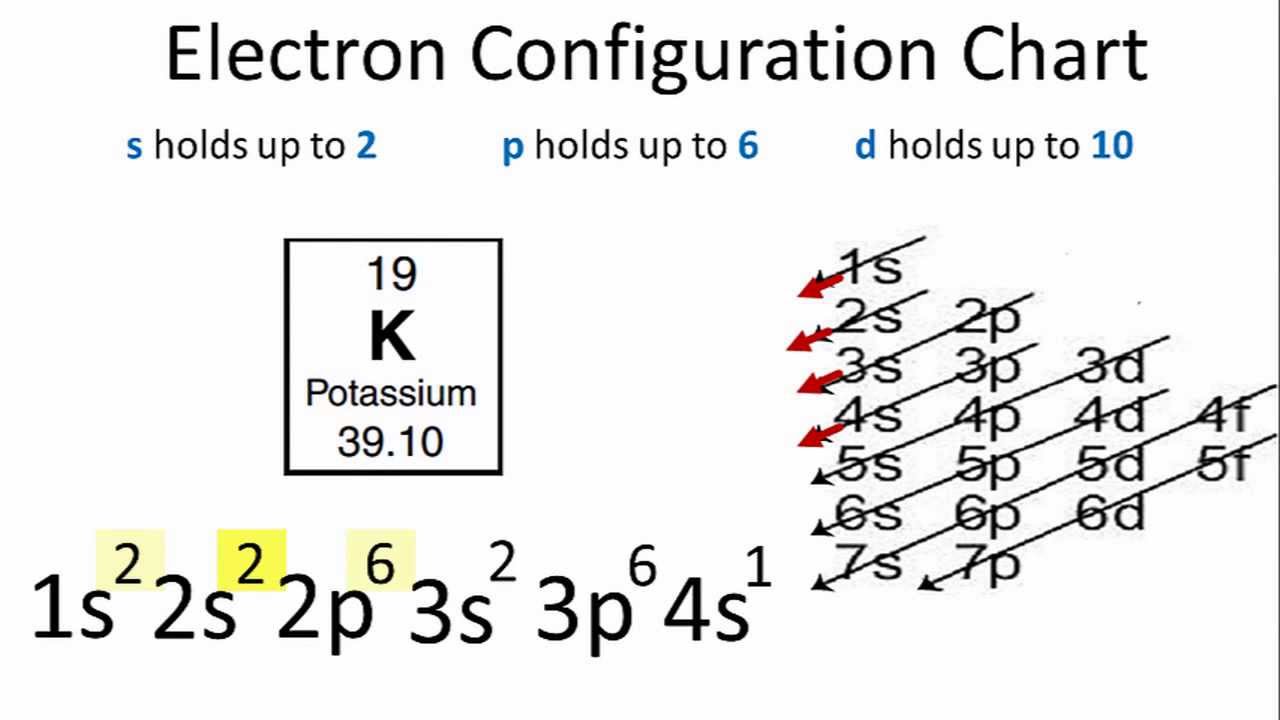
Cl-1 Valence Electrons




